Data Residency for Veeva CRM
Global compliance for Veeva PHI and CRM data

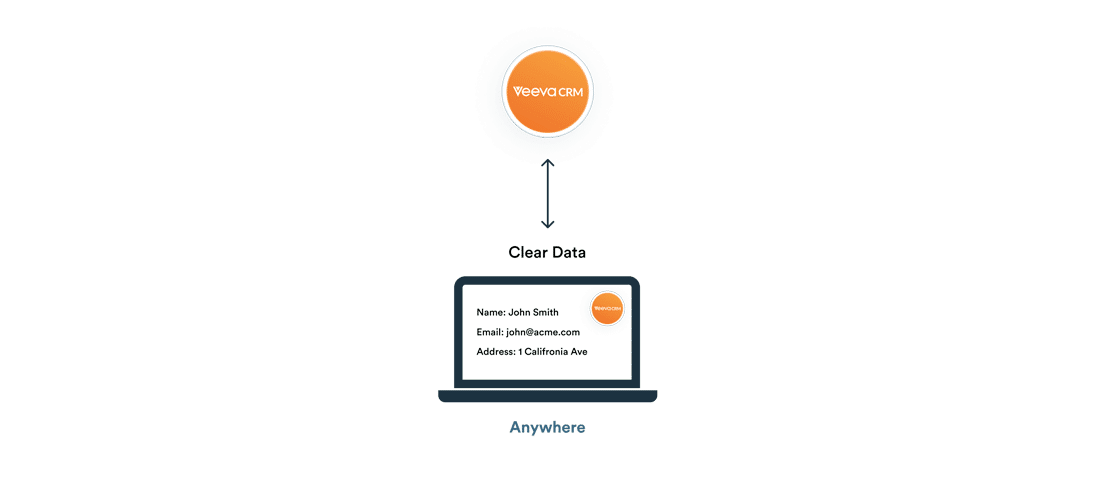
One World, One Veeva CRM
Run Veeva CRM applications with local compliance and data residency in China, Saudi Arabia, UAE, and more countries. Russia is supported only for life-saving medications in full compliance with OFAC and FZ-152.
Comparison of Solutions
- Category
- Veeva CRM Now
- Veeva CRM + InCountry
- Countries for data storage
- 3 countries
- Manage data across multiple countries in one instance
- No
- Single-tenant option
- No
- Worldwide*
- Yes
- Yes
* Worldwide other than Iran, North Korea, and Syria
InCountry Data Residency for Veeva CRM
Features
- HIPAA compliance
- Compliant with local regulatory requirements and data residency policies
- Store all data in a specific country depending on relevant regulatory and company requirements
Benefits
- Expand into new countries
- Enhanced value from Veeva solutions
- Mobile support
- Searchable protected data
- Reduced overall compliance risk
The InCountry Difference
How it Works
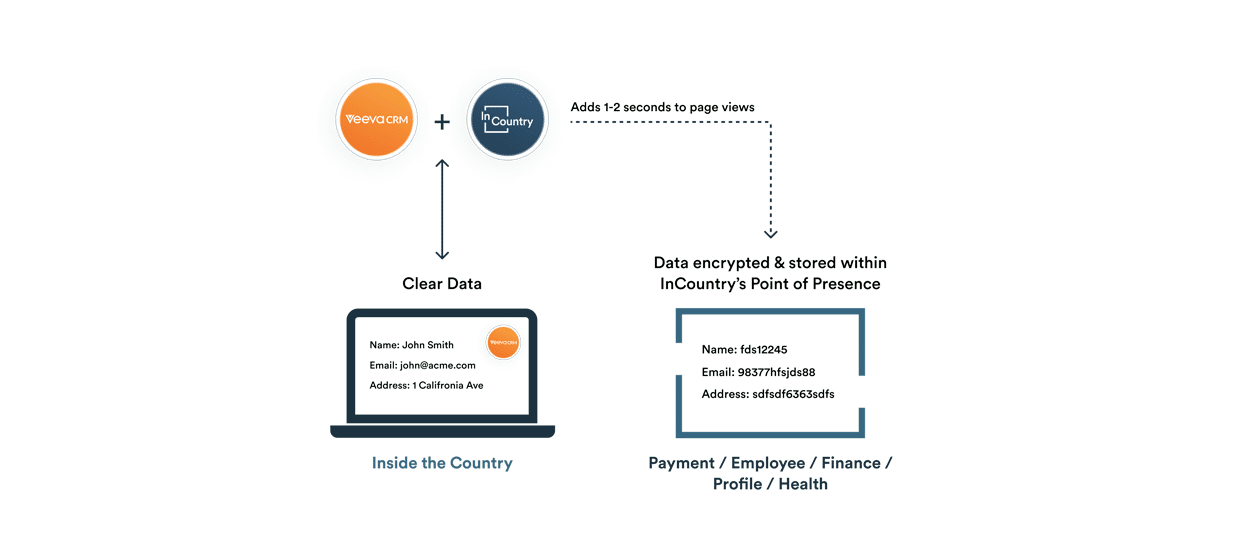
Augment existing Veeva forms to store administrator specified fields into InCountry data stores
InCountry traps all writes from the form and replaces existing fields with redacted data
Protected data can still be searched within Veeva
All data can be stored in a specific country, or logic can be applied to use an existing data field such as Country to direct storage to multiple countries
When Veeva reads the fields, they can be automatically replaced with the actual unredacted data when viewed in specific countries by Veeva users